Recently, the research team led by Professor Chi Yonggui, operating within Guizhou University's National Key Laboratory of Green Pesticides and Agricultural Engineering, has contributed two noteworthy projects to the international academic journal "Nature Communications." Their research efforts are predominantly focused on pioneering work in the field of organic catalytic synthesis and the advancement of environmentally friendly pesticides (as is seen in Figure 1).
Under the collaborative guidance of Professor Chi Yonggui and Professor Zheng Pengcheng, a team comprising Master's student Fan Guodong and Doctoral student Wang Qingyun, among others, has published a paper titled "Carbene-catalyzed chemoselective reaction of unsymmetric enedials for access to Furo[2,3-b]pyrroles." Additionally, Doctoral students Wang Qingyun and Wu Shuquan, together with their team, have contributed the paper "NHC-catalyzed enantioselective access to β-cyano carboxylic esters via in situ substrate alternation and release," revolving around the utilization of nitrogen heterocyclic carbene (NHC) as a pivotal biomimetic catalyst. This approach enables the precise manipulation of reaction sites and pathways by regulating various forms of chemoselectivity, ultimately facilitating the creation of chiral non-natural amino acids and furan-pyrrole type active compounds used in pharmaceuticals and pesticides.

Figure 1. Organic Catalytic Synthesis and Pharmaceutical Pesticide R&D
The first research endeavor, as illustrated in Figure 2, realized NHC-catalyzed asymmetric chemoselective control over enedials. It was established that chemoselectivity predominantly hinges on the oxidative-reductive attributes of the substrate/catalyst. The research team employed LC-HRMS characterization and DFT calculations to eliminate the influence of steric hindrance on selectivity within the reaction system. This approach empowers precise management of selective reactions among similar functional groups within compound molecules, yielding chiral Furo[2,3-b]pyrroles of remarkable optical purity. Furthermore, these chiral compounds have exhibited notable antibacterial activity against citrus canker bacteria.

Figure 2. Study on Chemoselectivity of Asymmetric Enedial Molecules and Biological Activity Testing
The second study, represented in Figure 3, introduced a strategy involving the use of distinct substrates for in-situ slow release. This strategy effectively diminishes the substrate concentration in the reaction system, thereby suppressing unwanted side reactions and ensuring critical control over chemoselective reaction pathways. Mechanistic investigations unveiled the capacity of the two substrates to partake in a pivotal reaction leading to the formation of a key intermediate. The presence of this intermediate plays a pivotal role in governing the substrate concentration within the system, effectively reducing the likelihood of undesired side reactions. The outcome of this process is the generation of chiral cyano carboxylic esters marked by high optical purity. Furthermore, this reaction can be scaled up to 5 grams (190-fold increase) while requiring a mere 0.5 mol% of the catalyst, a characteristic indicative of its potential industrial applicability. Subsequent derivatization studies have indicated that readily available γ-aminobutyric acid (GABA) class drugs such as propranolol, phenibut, and baclofen can be synthesized through a straightforward process.
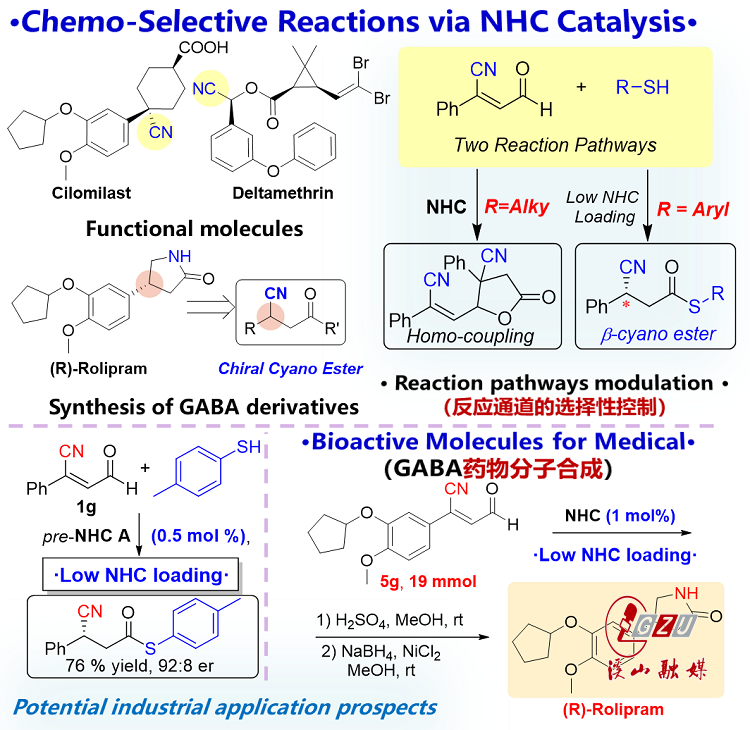
Figure 3. Investigation into NHC-Catalyzed Reaction Pathway Selectivity and GABA Drug Synthesis
Editor:Pang Aizhong, Han Xiaomei
Chief Editor: Li Xufeng
Senior Editor: Yan Nan
Translator: Xiao Qi